
Tanja Kovačević
PhD Candidate / NSF GRFP Fellow / H2H8 Fellow
Department of Earth and Planetary Science, University of California, Berkeley
email: tanja_kovacevic(at)berkeley(dot)edu
As a Ph.D. candidate in the Earth and Planetary Science Department (EPS) at U.C. Berkeley, my background includes a B.S. in Chemistry from CU Denver and the distinction of being a first-generation community college student. I am currently an NSF GRFP fellow and have used this opportunity to conduct research in the field of planetary science, specifically the study of planetary materials in the warm dense matter regime. I utilize ab initio molecular dynamics to determine conditions for rock-ice mixing with respect to super-earth and sub-neptune sized exoplanets.
In addition to my research, I am dedicated to fostering student engagement and outreach in the field of planetary science. I welcome any inquiries about my research or the field in general and am happy to share my experiences as a first-generation college student and community college transfer.
Outside of my academic pursuits, I enjoy spending time biking, practicing yoga, and knitting.
Research
The Mixing of MgO & H2O
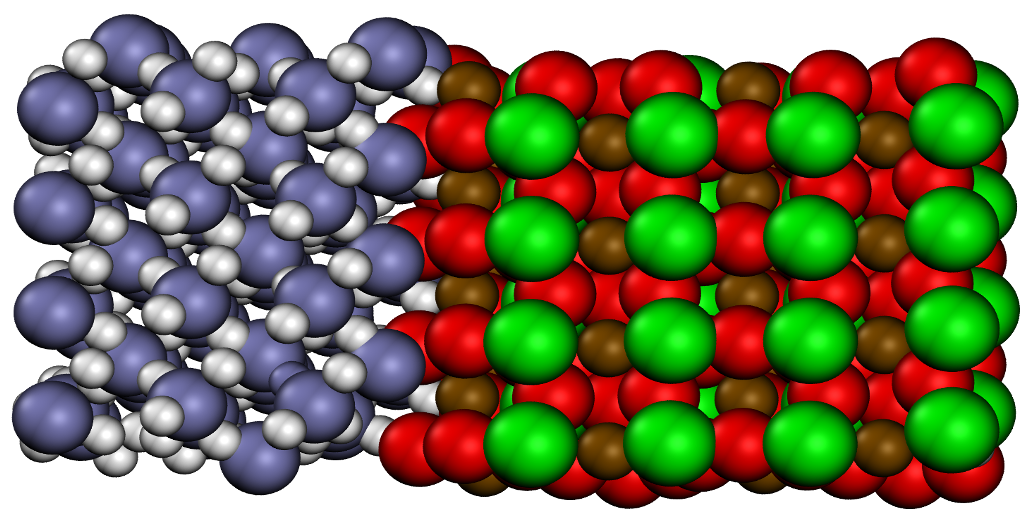
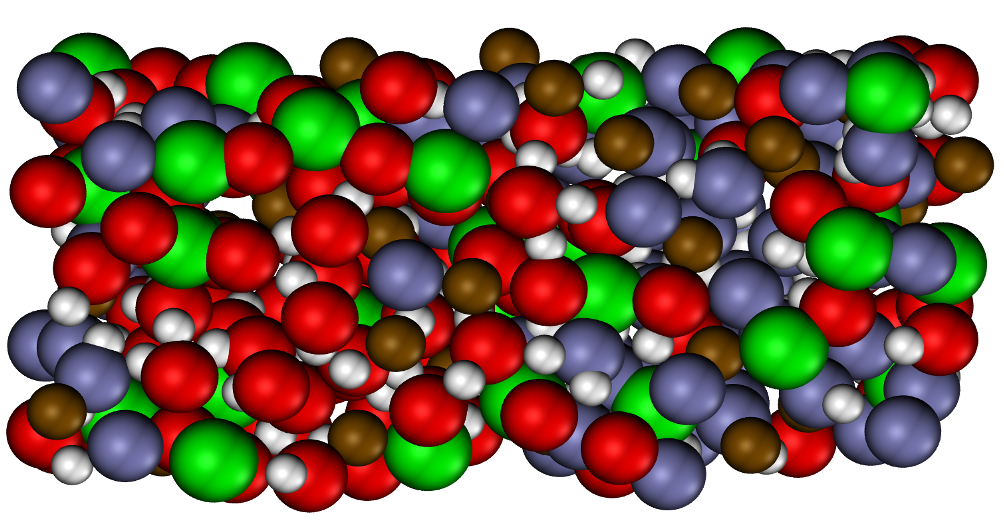
Investigating sub-Neptunes, exoplanets with a mass between Earth and Neptune, involves understanding their formation and evolution by examining their interior and constituent properties, especially those that may be water-rich. To shed light on the interaction between the rocky mantle and water-rich envelope, in this present article, we utilized density functional molecular dynamics simulations to explore the thermodynamic properties of MgO and H2O under extreme conditions. Employing a “heat-until-it-mixes” approach, we identified the pressure-temperature conditions at which rock and ice become fully miscible (the solvus point). Notably, we found that MgO and H2O mix well below MgO’s melt curve, which has significant implications for planetary evolution.These results support a hypothesis of compositional gradients within water-rich exoplanets, significantly influencing a planet’s evolution.



The mixing of MgSiO3 & H2O
Water worlds are exoplanets more massive than Earth that contain a significant amount of water overlaying a rocky mantle and iron core. Characterizing the interactions between water and rock under the pressures and temperatures within water worlds is essential to understanding their structure, formation, and evolution. In this article, we studied the dynamics between water and high-pressure MgSiO3, a major silicate phase, and determined the conditions when they form a homogeneous mixture. We find that MgSiO3 and H2O become miscible at the conditions found within the interiors of water-rich exoplanets during their collisional growth, forming a fuzzy, mixed layer and increasing the amount of water incorporated deep within the planet. A mixed layer affects chemical evolution and heat transport through a planet.

Publications
- T. Kovacevic, F. Gonzalez-Cataldo, B. Militzer, “The homogeneous mixing of MgO and H2O at extreme conditions“, Contrib. Plasma Phys. (2023) e202300017. DOI:10.1002/ctpp.202300017
- T. Kovacevic, F. Gonzalez-Cataldo, S. T. Stewart, B. Militzer, “Miscibility of rock and ice in the interiors of water worlds“, Scientific Reports 12 (2022) 13055. DOI:10.1038/s41598-022-16816-w
- T. Kovacevic, A. Skinner, J. Fisk, V. Fishback, S. Reed, “A semester-long, organic chemistry laboratory structured around unknown analysis and re-synthesis as a bridge to guided inquiry“, J. Chem. Ed. (2020) DOI: 10.1021/acs.jchemed.9b01037